|
Endovascular Treatment for Spontaneous bilateral carotid artery dissection with acute ischemic stroke: A Case Report with automated postprocessing CT perfusion findings and Review of the Literature
Authors:
Pao Sheng Yen, 1 , Yu-Hui Lin, 2 , Ting-Wei Chang, 3 , Chun-Pai Yang, 4 ,
1 Department of Neuroradiology, Kuang Tien General Hospital, Taichung, Taiwan
2 Department of Neurology, Kuang Tien General Hospital, Taichung, Taiwan
3 Department of Neuroradiology, Kuang Tien General Hospital, Taichung, Taiwan
4 Department of Neurology, Kuang Tien General Hospital, Taichung, Taiwan; Department of Nutrition, HungKuang University, Taichung, Taiwan.
Corresponding Author:
Pao Sheng Yen
keywords: Acute stroke, Bilateral Spontaneous carotid artery dissection, Endovascular treatment
Abstract
Carotid artery dissection (CAD) is a common cause of stroke, accounting for up to 25% of all ischemic strokes in young and middle-aged patients. CAD should be considered in young patients with unexplained head and neck pain, with or without focal neurological symptoms and signs. While the clinical features may raise suspicion for CAD, the diagnosis is confirmed by its typical neuroimaging findings. Meanwhile, simultaneous spontaneous dissection of the bilateral carotid artery has rarely been reported. We herein describe a clinically challenging case of a simultaneous bilateral CAD that was successfully treated with bilateral carotid artery stenting (CAS). The patient recovered satisfactorily after completing the whole course of treatment.
CASE HISTORY
A 49-year-old male triathlete with no known pre- existing medical conditions except for episodic neck pain presented in the emergency department for a 4-hour history of acute onset left-sided weakness that was associated with gait unsteadiness and dizziness. He was about to go swimming when he then developed right-sided nape and shoulder pain, which was soon followed by a sudden- onset slight left-sided weakness. The patient arrived at the emergency department approximately 4 hours after the onset of weakness. He was ambulatory upon presentation to the emergency department and had a calculated National Institutes of Health Stroke Scale (NIHSS) score of 0. An initial non-contrast head computed tomography (CT) scan was obtained and revealed no acute intracranial abnormality. Treatment with intravenous thrombolysis was considered in this patient who had ischemic stroke within 4.5 hours of the symptom onset, but this treatment was precluded because of an ineligible NIHSS score. With the working diagnoses of transient ischemic attack, cerebral artery dissection and cervical myelopathy, the patient was then admitted to the Neurology Ward 6 hours after the onset of left-sided weakness. On admission, the physical and neurological examinations were normal, and he had an admitting NIHSS score of 0. There was no evidence of Horner syndrome observed. Nevertheless, it is notable to mention that the patient frequently applied Gua Sha (a folk remedy that uses a blunt instrument to repeatedly scrape previously lubricated skin) to his neck every time he felt pain and stiffness in his neck. His medical, trauma and family histories were noncontributory. The cerebrovascular risk factors were unremarkable.
Three hours after admission to the neurology ward, his left-sided weakness suddenly deteriorated, at which time he almost fell while walking to the bathroom. His NIHSS score increased from a baseline of 0 to 8. Intravenous thrombolysis was reconsidered and precluded once again after judicious verification of his stroke onset time. Although too slight to be detected by the NIHSS, this man’s left-sided weakness never resolved to the normal premorbid state after the stroke onset. It would be discreet to consider this event as a stroke-in-evolution rather than a transient ischemic attack with a subsequent ischemic stroke. With the nape and shoulder pain occurring as the same time as the left hemiparesis, the patient needed further diagnostic testing for stroke etiologies that can present with neck pain and hemiparesis, such as aortic, carotid, or vertebral artery dissection. A contrast head and neck CT scan with automated postprocessing CT angiogram (CTA) and CT perfusion (CTP) by using commercially available software RapidAI (iSchemaView) was performed in this atypical presentation of stroke. The artificial intelligence (AI)-driven automated postprocessing software can automatically generate quantitative measures of patient’s Alberta Stroke Program Early CT Score (ASPECTS) on noncontrast CT, process CTP maps and detect large vessel occlusion (LVO) on CTA.
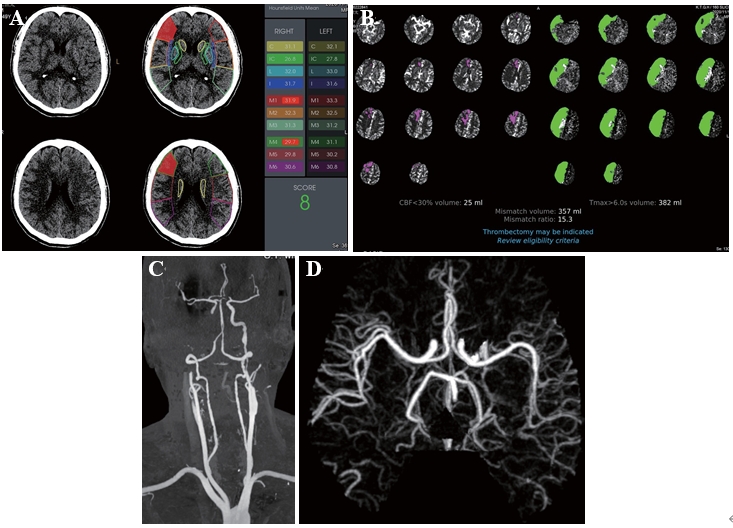
Figure 1. (A) Baseline axial CT performed 280 min after the symptom onset showed a faint hypodensity in the right frontal lobe with a RAPID-ASPECTS of 8. (B) The RAPID-CTP analysis demonstrated a complete infarct volume of 25 milliliters (mL), entailing a small infarcted core within a large volume of penumbra in the right internal carotid artery vascular territory. The configured Tmax > 6-second volume representing the combination of the infarcted core volume and hypoperfused brain was 382 mL in the right cerebral hemisphere, deriving a mismatch ratio of 15.3. (C) CTA showed a complete occlusion of the right cervical internal carotid artery (ICA). There was irregular stenosis and dilatation of the left ICA without occlusion. (D) RAPID-CTA showed no significant anomaly of the intracranial vessels.
The head CT scan showed no hemorrhage with an ASPECT score of 8/10 in the right middle cerebral artery (MCA) vascular territory (Figure 1a). The CTP analysis demonstrated a complete infarct volume of 25 milliliters (mL), entailing a small infarcted core within a large volume of penumbra in the right internal carotid artery vascular territory. The configured Tmax > 6-second volume representing the combination of the infarcted core volume and hypoperfused brain was 382 mL in the right cerebral hemisphere, deriving a mismatch ratio of 15.3 (Figure 1b). The CTA showed that there was a complete occlusion of the right cervical internal carotid artery (ICA) (Figure 1c, d). The 2019 AHA/ASA guidelines and emerging evidence support the benefit of mechanical thrombectomy in selected patients with acute ischemic stroke within 6 to 16 hours of last known normal who have a large vessel occlusion in the anterior circulation and meet the other DAWN or DEFUSE 3 eligibility criteria. Therefore, endovascular thrombectomy (EVT) should be considered for patients presenting with debilitating stroke caused by an occlusion of the internal carotid or the proximal middle cerebral arteries. Given this patient’s stroke onset time, clinical presentation and imaging findings, a multidisciplinary decision was made to initiate EVT.
The procedure was performed under general anesthesia. An 8 French long sheath introducer was placed in the right femoral artery. After a diagnostic cerebral angiographic study, a 7 French guiding catheter (Envoy, Codmen, USA) was inserted into the right proximal internal carotid artery. The right carotid injection demonstrated severe stenosis at the horizontal intrapetrous segment of the right ICA with an intramural hematoma due to the dissection (Figure 2). A 0.014-inch micro guidewire (Asahi Chikai black, Japan) was inserted carefully into the stenotic segment, and then exchanged with a 4 mm embolic protection device (SpiderFX, Medtronic, USA) into the right distal ICA. A 4 mm × 19 mm balloon- expandable stent (Integrity, Medtronic, USA) was placed into the stenosis and expanded slowly up to a pressure of 8 atmospheres; and then the balloon was deflated. After the placement of the first stent, the control right carotid angiogram revealed displacement of the intramural hematoma caudally to the ascending intrapetrous portion causing stenosis. Another 4 mm × 19 mm balloon- expandable stent (Integrity®, Medtronic, USA) was then placed into the stenotic segment to partially overlap the first segment. Post-stenting right carotid angiogram revealed complete restoration of the stenosis and re- establishment of the normal intracranial flow (Figure 3). The subsequent left carotid angiogram demonstrated a focal dilatation at the distal cervical segment of the left ICA, suggesting a pseudoaneurysm (Figure 4). Because there was no significant cerebral flow compromise, carotid stenting was not performed at that time.
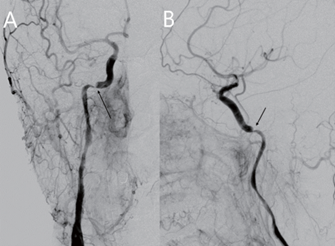
Figure 2. Digital subtraction angiography. Right common carotid artery (CCA) injection, (A) anteroposterior projection and (B) lateral projections demonstrating severe segmental stenosis at the horizonal intrapetrous portion of the internal carotid artery (ICA) due to the dissection with an intramural hematoma (arrow).
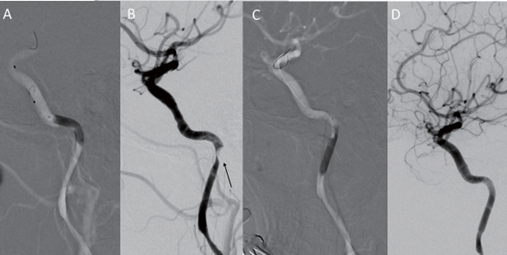
Figure 3. Digital subtraction angiography. Right internal carotid artery (ICA) injection, lateral projection (A) After placement of the first stent, the intramural hematoma was displaced caudally to the ascending intrapetrous portion. (D) After placement of the second stent, the stenotic segment was completely restored.
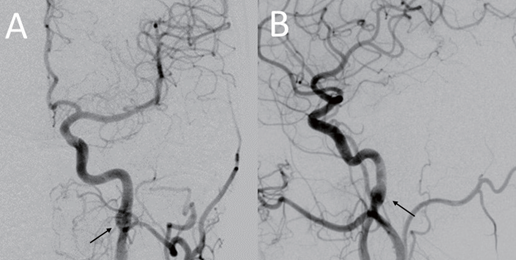
Figure 4. Digital subtraction angiography. Left common carotid artery (CCA) injection, (A) anteroposterior projection and (B) lateral projection demonstrating focal dilatation at the distal cervical ICA (arrows) without compromising blood flow, which was suspect due to the pseudoaneurysm formation.
After awakening from anesthesia, the patient’s neurological examination was normal, and he had a significant improvement in his NIHSS score from a preprocedural score of 8 to a postprocedural score of 0. Dual-antiplatelet therapy was initiated by a loading dose of 300 mg clopidogrel (Plavix) and 100 mg aspirin. The postprocedural MRI on the next day revealed several diffusion-weighted imaging (DWI) hyperintense spots in the frontal lobes bilaterally, which was characterized by watershed-type infarcts in the right centrum semiovale (Figure 5). The major intracranial blood vessels appeared patent on magnetic resonance angiography (MRA).
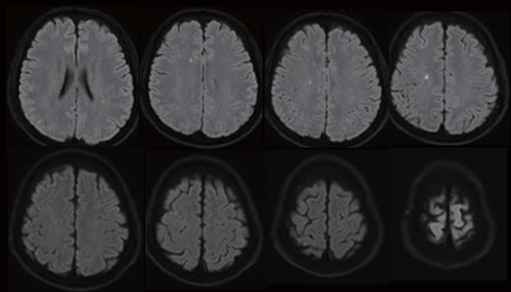
Figure 5. MRI with diffusion-weighted scan after stenting revealed several tiny hyperintense spots in the bifrontal centrum semiovale suggestive of acute watershed infarcts.
Nevertheless, 2 days after the uneventful endovascular intervention, this patient reported a newly developed visual disturbance in his left eye, and he had a NIHSS of 0. Due to the possibility of a recurrent thromboembolic event, a follow-up contrast head and neck CT scan with automated postprocessing CTA and CTP was once again obtained. Paradoxically, the RAPID CTP analysis revealed that there was a substantial region of penumbra in the left middle cerebral artery vascular territory without an apparent infarcted core (Figure 6). Congruently, the CTA revealed an occlusion of the extracranial right ICA. Cerebral angiography study was performed under local anesthesia via the right femoral artery approach. An injection into the left carotid via a 7 French guiding catheter (Envoy, Codmen, USA) revealed severe segmental stenosis at the distal ICA just below the pseudoaneurysm due to further dissection of the vessel. Under roadmap guidance, a 4 mm embolic protection device (SpiderFX, Medtronic, USA) was deployed in the distal ICA followed by the placement of a 5 mm × 30 mm and a 7 mm × 50 mm Wallstent over the stenotic segment. Moreover, another 4 mm × 19 mm balloon-expandable overlapping stent (Integrity®, Medtronic, USA) was placed between the gaps of the Wallstents to completely cover the dissected vessel (Figure 7). Post-stenting left carotid angiogram showed that there was an immediate restoration of the normal vessel diameter and normal cerebral perfusion. Postoperative co urse was uneventful, and the patient was discharged home with a complete resolution of his neurological deficits. Follow-up CTA three months later revealed that the carotid arteries were patent bilaterally (Figure 8).
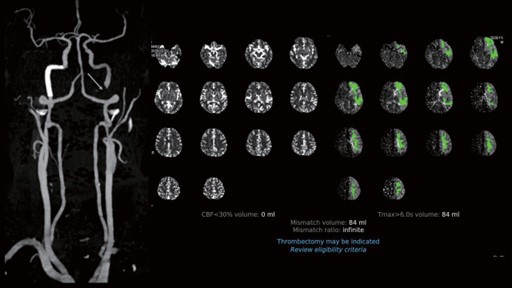
Figure 6. The RAPID CTP analysis revealed a substantial region of the penumbra in the left middle cerebral artery vascular territory without an apparent infarcted core.
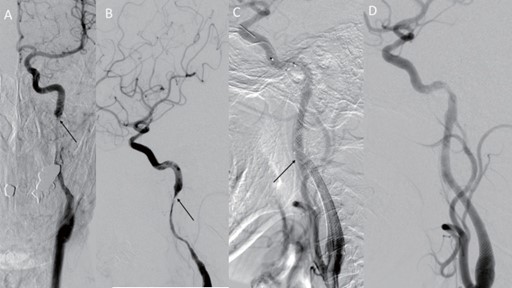
Figure 7. Left common carotid artery (CCA) injection, (A) anteroposterior projection and (B) lateral projection demonstrated severe segmental stenosis at the distal ICA just below the pseudoaneurysm (arrow). (C) Under roadmap guidance, a 4 mm embolic protection device (SpiderFX, Medtronic, USA) was deployed in the distal ICA. A 5 mm x 30 mm and a 7 mm x 50 mm Wallstent were advanced without prior dilation and were deployed over the stenosis. However, there was a gap between the stents (arrow), and another 4 mm x 19 mm balloon-expandable overlapping stent (Integrity®, Medtronic, USA) was placed in between for completely cover the dissected vessel. (D) Poststenting left carotid angiograms showed an immediate restoration of the normal vessel diameter and cerebral perfusion.
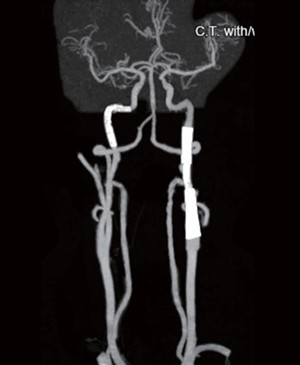
Figure 8. CTA at the 3-month follow-up showed a widely patent stent with good cerebral perfusion.
DISCUSSION
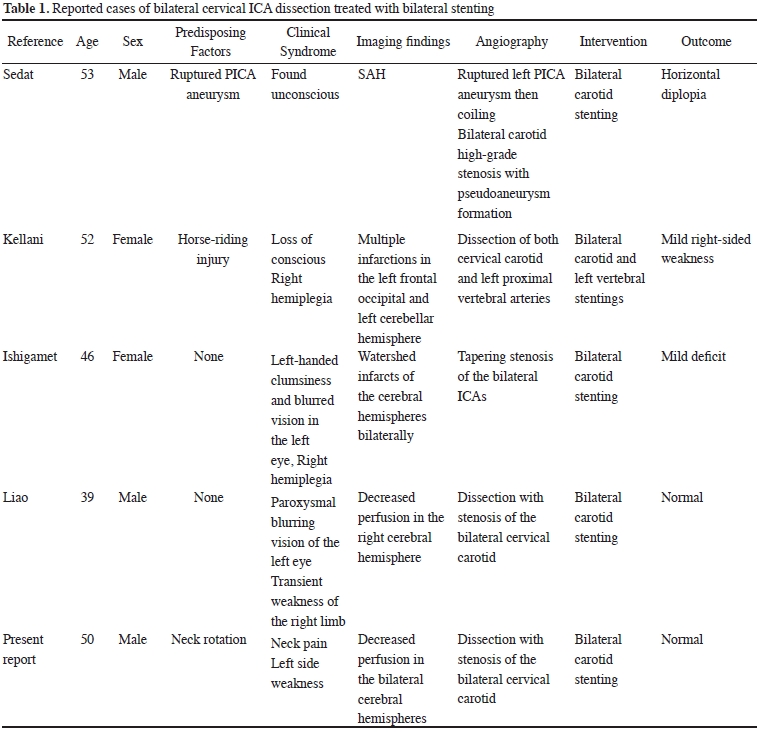
Carotid artery dissection is the most common cause of ischemic stroke in younger patients (younger than 50 years old). Ischemic stroke can be caused by major trauma as well as relatively minor incidents such as sneezing, coughing, sports, or chiropractic manipulation. In the absence of a significant inciting traumatic event, ischemic stroke is generally recognized as a spontaneous carotid artery dissection (SCAD) (1). Most of the reported SCAD cases are unilateral, while bilateral involvement only accounts for approximately 10% of all SCAD patients. The causative factors for spontaneous bilateral internal carotid artery dissection are still unknown. The incidence of dissection is increased in the settings of migraine, hypertension, and smoking, and in patients on oral contraceptives. A few connective tissue disorders, including fibromuscular dysplasia, Ehlers–Danlos syndrome and Marfan's syndrome, have been reported to be associated with SCAD, even in the setting of known minor trauma, suggesting that abnormalities in the connective tissue surrounding the vessels can increase the vulnerability of these patients to injury (2). Arterial dissection results from tears of the intimal layers and the subsequent leakage of the blood into the vessel wall, causing mural hematomas. These mural hematomas can cause neurologic sequelae due to the compromise of the blood flow by narrowed or occluded vessels or by a thromboembolism (3,4). The sudden onset of arterial dissection involves an instantaneous hemodynamic compromise that is more severe than that in atherosclerotic lesions, as the collateral circulation has not yet sufficiently developed (5). Most ischemic cerebral symptoms arise from thromboembolic mechanisms and are adequately treated with anticoagulation or antiplatelet medications. SCAD-related acute ischemic strokes can be severe and life-threatening, and the quality of life of patients with SCADs may be impaired in most survivors after dissection (6,7). The efficacy of EVT in treating patients with strokes related to SCAD is still sparsely studied and unproven (8). However, patients presenting with acute hemodynamic insufficiency can be managed with emergent stent placement to restore the patient’s cerebral perfusion, provided that irreversible infarction has not already occurred. The prevention of stroke is the main concern in SCAD treatment. The ideal timing of carotid stenting and the optimal antithrombotic strategy in this setting remain controversial. Emergent carotid stenting itself could increase the risk of thromboembolic complications. Additionally, antithrombotic drugs are required during and after the procedure, which might increase the risk of postoperative hemorrhagic complications, especially in severe stroke patients with low ASPECTS and who are being treated with rt-PA (recombinant tissue plasminogen activator). Retrospective studies have suggested that the risk of symptomatic ICH ranges from 9% to 20% in the retrieve-and-stent approach (9). In contrast, the retrieve- and-wait method may lead to an unacceptably high risk of recurrent disabling stroke (10,11). Nevertheless, in multiple reported series, stenting for SCAD was found to be safe and effective. The indications for carotid stenting in acute SCAD include the failure of medical therapy, the presence of a new ischemic event, the progression of neurologic symptoms, evidence of symptomatic perfusion deficits by perfusion studies and an enlarging pseudoaneurysm (12,13). The treatments of simultaneous bilateral ICA dissections have been reported in very few cases due to the lack of indications and various limitations. These cases are summarized in Table 1 (14).
Some technical tips that need to be kept in mind during stenting include the use of embolic protection systems, the meticulous navigation of the microguidewire through the stenotic area into the true lumen, and the selection of the proper type of stent for deployment. In our case, more flexible balloon-expandable stents rather than self-expanding stents (Wallstent) were used in the intrapetrous portion of the internal carotid artery. Surprisingly, the intramural hematoma could be displaced easily during stent deployment.
CONCLUSION
We have encountered a case of bilateral ICAD that was successfully treated with simultaneous bilateral CAS. A simultaneous spontaneous dissection of both carotid arteries is rarely reported, but it is a noteworthy cause of ischemic stroke, particularly in young patients with even minor trauma. Recent advances in noninvasive imaging technologies such as the Rapid AI have made the diagnosis much easier. For patients with CAD presenting with acute hemodynamic insufficiency, such as in patients with a TIA or an acute ischemic stroke, regardless of the prior rt-PA treatment, a timely mechanical thrombectomy is indicated in selected SCAD patients when this technique is performed at stroke centers with providers who have expertise. An angioplasty and the emergency stenting of CAD may be the treatment options in addition to mechanical thrombectomy at expert centers. However, the long-term benefits of endovascular CAS remain to be determined.
REFERENCES
- Engelter ST, Grond-Ginsbach C, Metso TM, Metso AJ, Kloss M, Debette S, Leys D, Grau A, Dallongeville J, Bodenant M, Samson Y, Caso V, Pezzini A, Bonati LH, Thijs V, Gensicke H, Martin JJ, Bersano A, Touzé E, Tatlisumak T, Lyrer PA, Brandt T; Cervical Artery Dissection and Ischemic Stroke Patients Study Group. 2013 21; 80(21): 1950-7.
- Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J 2001 22; 344(12):898-906.
- Blunt SB, Galton Cervical carotid or vertebral artery dissection. BMJ. 1997 25; 314(7076):243.
- Benninger DH, Georgiadis D, Kremer C, Studer A, Nedeltchev K, Baumgartner R Mechanism of ischemic infarct in spontaneous carotid dissection. Stroke 2004; 35: 482-485.
- Morel A, Naggara O, Touzé E, Raymond J, Mas JL, Meder JF, Oppenheim Stroke. 2012; 43: 1354- 1361.
- Engelter ST, Rutgers MP, Hatz F, Georgiadis D, Fluri F, Sekoranja L, Schwegler G, Müller F, Weder B, Sarikaya H, Lüthy R, Arnold M, Nedeltchev K, Reichhart M, Mattle HP, Tettenborn B, Hungerbühler HJ, Sztajzel R, Baumgartner RW, Michel P, Lyrer PA. Intravenous thrombolysis in stroke attributable to cervical artery dissection. Stroke 2009; 40: 3772-3776.
- Rubiera M, Ribo M, Delgado-Mederos R, Santamarina E, Delgado P, Montaner J, Alvarez-Sabín J, Molina CA. Tandem internal carotid artery/middle cerebral artery occlusion: an independent predictor of poor outcome after systemic thrombolysis. Stroke. 2006;37(9): 2301-2305.
- Farouk M, Sato K, Matsumoto Y, Tominaga Endovascular treatment of internal carotid artery dissection presenting with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2020 ; 29: 104592.
- Kim Y, Choi CH, Lee TH, Cho HJ, Sung SM, Baik SK, Ko JK. Endovascular stenting for symptomatic carotid dissection with hemodynamic World Neurosurg 2017; 102: 598-607
- Benninger DH, Georgiadis D, Kremer C, Studer A, Nedeltchev K, Baumgartner R Mechanism of ischemic infarct in spontaneous carotid dissection. Stroke 2004; 35: 482-485.
- Morel A, Naggara O, Touzé E, Raymond J, Mas JL, Meder JF, Oppenheim Mechanism of ischemic infarct in spontaneous cervical artery dissection. Stroke 2012; 43: 1354-1361.
- Cohen JE, Leker RR, Eichel R, Gomori M, Itshayek Emergency endovascular revascularization of tandem occlusions: Internal carotid artery dissection and intracranial large artery embolism. J Clin Neurosci 2016; 28: 157-161.
- Malek AM, Higashida RT, Phatouros CC, Lempert TE, Meyers PM, Smith WS, Dowd CF, Halbach Endovascular management of extracranial carotid artery dissection achieved using stent angioplasty. AJNR Am J Neuroradiol 2000; 21:1280-1292.
- Liao M, Chen X, Chen H, Wang Y, Zeng J, Fan Y. Stent-Assisted Angioplasty in Spontaneous Bilateral Extracranial Internal Carotid Artery Dissection, Front Neurol. 2020; 11: 582253.
|